Definition of boiling point pdf
Definition of BOILING POINT: The temperature at which a liquid exposed to the atmosphere begins to boil. This temperature varries with barametric pressure of the atmosphere. This temperature varries with barametric pressure of the atmosphere.
Again, at a certain temperature called the boiling point, the molecules will gain enough energy to break free and become a gas. The boiling point for water is 100 degrees C (212 degrees F). The boiling point for water is 100 degrees C (212 degrees F).
the boiling point of a liquid at a pressure of 14.7 pounds per square inch absolute (psia). This pressure is equivalent to 760 millimeters of mercury (760 mm Hg). Attemperaturesabove the boiling point, the pressure of the atmosphere can no longer hold the liquid
boiling point n. 1. Abbr. BP a. The temperature at which a liquid boils at a fixed pressure, especially under standard atmospheric conditions. b. The temperature at which the vapor pressure of a liquid is equal to the ambient atmospheric pressure. 2. Informal a. The point at which one loses one’s temper. b. The point of crisis; the turning
The boiling point of a substance is the temperature at which it can change its state from a liquid to a gas throughout the bulk of the liquid. A liquid may change to a gas at temperatures below
Point. A distinct proposition or Question of Law arising or propounded in a case. In the case of shares of stock, a point means . In the case of bonds a point means , since a bond is quoted as a percentage of ,000.
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapor pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding environmental pressure.
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
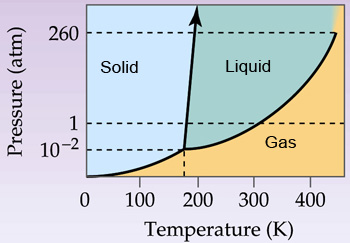
Boiling Point – Saturation In thermodynamics, the term saturation defines a condition in which a mixture of vapor and liquid can exist together at a given temperature and pressure. The temperature at which vaporization (boiling) starts to occur for a given pressure is called the saturation temperature or boiling point .
boiling point (countable and uncountable, plural boiling points) ( physics , chemistry ) The temperature at which a liquid boils , with the vapor pressure equal to the given external pressure. ( idiomatic ) The state of being heated , with high aggression .
Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. The temperature at which a solid and liquid phase may coexist in equilibrium. The term applies to pure liquids
The boiling point of a compound is the temperature at which it changes from a liquid to a gas. This This is a physical property often used to identify substances or to check the purity of the compound.
1 the temperature at which a liquid starts to boil The boiling point of water is 100°C. Bring the sauce to boiling point. Oxford Collocations Dictionary verb + boiling point …
Quiz & Worksheet Boiling Definition in Chemistry Study.com
boiling-point noun Definition pictures pronunciation
called fractional distillation. In this experiment you will be separating a mixture In this experiment you will be separating a mixture of two liquids with similar boiling points – ethanol and water.
2.2.4 Estimating the Relative Volatility From Boiling Point Data The Clapeyron equation relates the vapour pressure temperature dependency to the specific heat of vaporization ( ) and volume change between liquid and
Boiling point is also known as saturation temperature. Sometimes boiling point is defined by the pressure at which the measurement was taken. In 1982, the International Union of Pure and Applied Chemistry (IUPAC0 defined the standard boiling point as the temperature of boiling under 1 bar of pressure. The normal boiling point or atmospheric boiling point is the temperature at which the …
Fractional Distillation Definition. Fractional distillation is a process by which components in a chemical mixture are separated into different parts (called fractions) according to their different boiling points.
The boiling point of a substance is the temperature at which it changes state from liquid to gas throughout the bulk of the liquid. At the boiling point molecules anywhere in the liquid may be vaporized. The boiling point is defined as the temperature at which the saturated vapor pressure of a
Definition of boiling point The temperature at which the vapor pressure of a liquid is equal to the applied pressure; also the condensation point Search the Dictionary for More Terms
boiling point the temperature at which a liquid will boil; at sea level the boiling point of water is 100°C (212°F). cardinal p’s 1. the points on the different refracting media of the eye that determine the direction of the entering or emerging light rays.
Boiling point Freezing point Osmotic pressure. Learning objectives Describe meaning of colligative property Use Raoult’s law to determine vapor pressure of solutions Describe physical basis for vapor pressure lowering Predict magnitude of vapor pressure lowering based on chemical formula Calculate osmotic pressure in solution and use to determine molar mass of solute Predict direction of
A stricter definition of boiling point is the temperature at which the liquid and vapor (gas) phases of a substance can exist in equilibrium. When heat is applied to a liquid, the temperature of the liquid rises until the vapor pressure of the liquid equals the pressure of the surrounding atmosphere (gases).
A minimum boiling azeotrope is a liquid mixture that has a lower boiling point than its individual parts. Azeotropes, also called constant boiling mixtures, are combinations made up of two or more liquids whose chemical makeup cannot be changed by distillation.
English Language Learners Definition of boiling point : the temperature at which a liquid begins to boil : the point at which people might do or say something violent or might take some definite or extreme action because of anger, disagreement, etc.
DETERMINATION OF BOILING POINTS. Introduction: The boiling point of a compound is the temperature at which it changes from a liquid to a gas. This is a physical property often used to identify substances or to check the
point of a solid and the boiling point of a liquid. These methods will be needed again in several more These methods will be needed again in several more experiments in the future ( e.g . if you make a solid compound, you should record the melting point as a
The boiling point of a liquid is the temperature at which it starts to change into steam or vapor. For example, the boiling point of water is 212° Fahrenheit. Heat the cream to boiling point and pour it over the chocolate. 2. uncountable noun If a situation reaches boiling point or the boiling
Boiling point: The boiling point of a liquid is defined as the temperature at which the vapour pressure of the liquid is equal to the atmospheric pressure. We also know that the vapour pressure of any solvent decreases when some amount of solute is added to it.
boiling point, temperature at which a substance changes its state from liquid to gas. A stricter definition of boiling point is the temperature at which the liquid and vapor (gas) phases of a substance can exist in equilibrium.
the boiling point is less as informative criterion for the purity or for the description of materials as the melting point. Melting Point Laboratory guide 7
Another definition involves the concept of vapor pressure and states that the boiling point is the temperature at which the vapor pressure of the liquid is equal to the surrounding pressure. More details are provided in Subsection 4.6.5 .
boiling point – the temperature at which a solid turns into a liquid 186 were donated in November This month, we are on track to donate 198 home recent additions webmaster page banners feed a child
Boiling point ScienceDaily
DISTILLATION OF LIQUID FUELS BY THERMOGRAVIMETRY He Huang, Keyu Wang, Shaojie Wang, M definition (7). the IBP (Initial Boiling Point) is the temperature detected as the first drop of condensate falls into the distillate receiver. Thus, in this TG method, the Initial Boiling Point was the temperature recorded at which the distillate mass is above zero. The mass of the fust drop of the
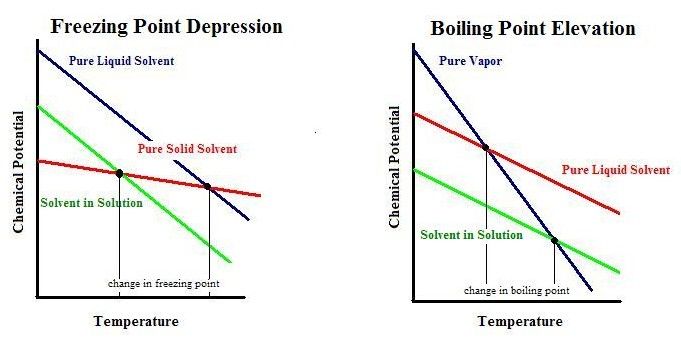
Boiling point elevation, like freezing point depression, is a colligative property of matter. This means it depends on the number of particles present in a solution and not on the type of particles or their mass.
Definition of BOILING POINT: The temperature when a liquid turns into a gas. For water is is 100 degrees celcuis. A solvent based liquid has a lower boiling point. Flammable liquids For …
Boiling, the cooking of food by immersion in water that has been heated to near its boiling point (212 °F [100 °C] at sea level; at higher altitudes water boils at lower temperatures, the decrease in boiling temperature being approximately one degree Celsius for each 1,000 feet [300 metres]).
Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equalled by the pressure exerted by the vapour of the liquid; under this condition, addition of heat results in the transformation of the liquid into its vapour without raising the temperature.
The increase in boiling temperature is due to pure ethylene glycol having a much higher boiling point and lower vapor pressure than pure water; there is no chemical stabilization against boiling of the liquid phase at intermediate compositions, as there is against freezing.
the point beyond which one becomes visibly angry, outraged, or the like: [usually singular] I had reached my boiling point with those nasty kids. the point at which matters reach a crisis: [ usually singular ] The situation had reached the boiling point between the two countries.
the high boiling point of water. Interaction between Induced Electric Dipoles In all polar and non-polar atoms and molecules, the valence electron charge distribution permanently fl uctuates due to interactions between the molecules with each other as well as electromagnetic radiation. As a consequence, tem- porary electric dipoles are steadily formed. These temporary dipoles interact with – crown hot water boiler manual Search boiling point and thousands of other words in English definition and synonym dictionary from Reverso. You can complete the definition of boiling point given by the English Definition dictionary with other English dictionaries: Wikipedia, Lexilogos, Oxford, Cambridge, Chambers Harrap, Wordreference, Collins Lexibase dictionaries, Merriam Webster…
Boiling Point definition, categories, type and other relevant information provided by All Acronyms. BP stands for Boiling Point BP stands for Boiling Point Search for acronyms, abbreviations,
Knowledge application – use your knowledge to identify the definition of boiling point Additional Learning . Take a look at more on this subject by reviewing the lesson named Boiling Definition in
19/06/2013 · Definition of Boiling Point The temperature at which a liquid boils and turns into a gas. The boiling point temperature will be lower if the atmospheric pressure is decreased.
A change in the value of a security or a security index or average. For common and preferred stocks a point represents a change of . For bonds a point represents a 1% change in face value.
The temperature at which a liquid changes to a vapor or gas. As the temperature of a liquid rises, the pressure of escaping vapor also rises, and at the boiling point the pressure of the escaping vapor is equal to that exerted on the liquid by the surrounding air, causing bubbles to form.
Vapor Pressure and Boiling Point The boiling point of a liquid is defined as that temperature when the vapor pressure of the liquid is equal to the total pressure of gas over the liquid The normal boiling point is defined as that temperature when the vapor pressure of the liquid is equal to 1 atm For H 2 O, the normal boiling point is 100.0 oC For CH 3 OH, the normal boiling point is 64.6 oC
The freezing point depression of a solution is a colligative property of the solution which is dependent upon the number of dissolved particles in the solution. The higher the solute concentration, the greater the freezing point depression of the solution. The freezing point plot of a pure solvent and a solution are shown below: The freezing point of the pure solvent is at constant temperature
Difference Between Vapor Pressure and Boiling Point Definition. Vapor Pressure: Vapor pressure is the force exerted by the vapor released by a liquid or solid substance in a closed container or space. Boiling Point: Boiling point is the temperature at which the vapor pressure is equal to the external pressure applied on the liquid. Specific Conditions. Vapor Pressure: Vapor pressure is defined
Boiling Point is the temperature level at which vapor pressure of a liquid rises and equals the pressure surrounding the liquid causing it to change into vapor.
Boiling point definition, the temperature at which the vapor pressure of a liquid is equal to the pressure of the atmosphere on the liquid, equal to 212°F (100°C) for water at …
boiling (boyl′īng) Process of vaporizing a liquid. Boiling water destroys most microorganisms (but may not destroy spores or viruses), solidifies (denatures) albumin, weakens fibrin and muscle proteins in meat, bursts starch granules, and softens cellulose in cereals and vegetables. boiling bringing to the boil. boiling fowl a mature hen of
The melting point of a pure compound is an intensive property, like density and boiling point. Intensive properties are independent of the amount of substance present. The melting point of a compound is the temperature at which it changes from a solid to a liquid. Experimentally, melting point is actually recorded as the
@user185991, at the boiling point (leave out the word “normal”), the vapor pressure of water is equal to the ambient pressure. If you have a beaker of water under a bell jar, and expose it to vacuum, the ambient pressure is vacuum pressure, not atmospheric pressure.
Boiling point is the temperature at which the vapor pressure of a liquid equals the pressure of the atmosphere. But caution must be taken since temperature itself in thermodynamics is a state variable and is defined, by the first approach, in a equilibrium condition.
Boiling or the boiling point is the temperature when a liquid will have vaporization occurring through the entire liquid (not just on the surface). This occurs when the liquid’s vapor pressure
Boiling Point Elevation. If addition of a nonvolatile solute lowers the vapor pressure of the solution via Raoult’s law , then it follows that the temperature must be raised to restore the vapor pressure to the value corresponding to the pure solvent.
Antimony Melting Point – Boiling Point – Nuclear Power
Definition of boiling_point Chemistry Dictionary

Boiling pointDefinition Elevation in boiling point
thermodynamics Confusion about the Definition of Boiling
Boiling cooking Britannica.com
FlammableLiquids 29 CFR 1910
What is BOILING POINT? definition of BOILING POINT
boiling point definition-of.com
boiler operation manual free download – Potassium Melting Point – Boiling Point – Nuclear Power
8.4 Colligative Properties Boiling Point Elevation and

What is Boiling Point? Definition from Petropedia
Solvents and solubilities MicroChemicals GmbH
9 replies on “Definition of boiling point pdf”
Leave a CommentA change in the value of a security or a security index or average. For common and preferred stocks a point represents a change of . For bonds a point represents a 1% change in face value.
FlammableLiquids 29 CFR 1910
Boiling point definition of boiling point by The Free
Boiling point Wikipedia
The freezing point depression of a solution is a colligative property of the solution which is dependent upon the number of dissolved particles in the solution. The higher the solute concentration, the greater the freezing point depression of the solution. The freezing point plot of a pure solvent and a solution are shown below: The freezing point of the pure solvent is at constant temperature
boiling point definition-of.com
FlammableLiquids 29 CFR 1910
Quiz & Worksheet Boiling Definition in Chemistry Study.com
A stricter definition of boiling point is the temperature at which the liquid and vapor (gas) phases of a substance can exist in equilibrium. When heat is applied to a liquid, the temperature of the liquid rises until the vapor pressure of the liquid equals the pressure of the surrounding atmosphere (gases).
Boiling point definition and meaning Collins English
boiling point Wiktionary
Boiling cooking Britannica.com
point of a solid and the boiling point of a liquid. These methods will be needed again in several more These methods will be needed again in several more experiments in the future ( e.g . if you make a solid compound, you should record the melting point as a
Definition of boiling_point Chemistry Dictionary
Melting and Boiling Points of Matter Ducksters
Definition of boiling point The temperature at which the vapor pressure of a liquid is equal to the applied pressure; also the condensation point Search the Dictionary for More Terms
Kids science Melting and Boiling Ducksters
point of a solid and the boiling point of a liquid. These methods will be needed again in several more These methods will be needed again in several more experiments in the future ( e.g . if you make a solid compound, you should record the melting point as a
Boiling Point Elevation Definition and Process ThoughtCo
boiling point definition English definition dictionary
Boiling Point definition, categories, type and other relevant information provided by All Acronyms. BP stands for Boiling Point BP stands for Boiling Point Search for acronyms, abbreviations,
Boiling point definition of boiling point by The Free
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
Boiling point definition and meaning Collins English
Point. A distinct proposition or Question of Law arising or propounded in a case. In the case of shares of stock, a point means . In the case of bonds a point means , since a bond is quoted as a percentage of ,000.
Difference Between Vapor Pressure and Boiling Point
Comments are closed.