Organic compounds boiling point pdf
The boiling points of carboxylic acids are high relative to the other organic compounds we have discussed (Table 11.3). Carboxylic acids interact very strongly by forming hydrogen-bonded dimers. These dimers have higher
More Essay Examples on London Rubric. There are three trends that affect the boiling and melting points and these are the following: The relative strength of the four intermolecular attractions is factor significally affects the boiling point and the melting point of a compound.
And that’s reflected in the higher boiling point for 3-hexanol, right? 3-hexanol has a higher boiling point than 3-hexanone and also more than hexane. So when you’re trying to figure out boiling points, think about the intermolecular forces that are present between two molecules. And that will allow you to figure out which compound has the higher boiling point.
The boiling point of the compound is shown in the text box. You can select the actual compound from the ‘Select the actual compound’ drop down list. You can verify your result by clicking on the ‘Show chart’ button.
The boiling point, ranging from 175-600 °C, and carbon chain length, 9 to 70 atoms, of the fuel increases with fuel oil number. Viscosity also increases with number, and the heaviest oil has to be heated in order to get into liquid state.
Boiling Points of Organic Compounds As more carbons are added to a carboxylic acid, the trend indicates that the boiling point increases. The main functional group in a carboxylic acid (COOH) leads to strong bonds to start off with.
Using the above relationships, the boiling and melting points of nearly 1,000 non-hydrogen-bonding organic compounds have been correlated. The correlations for boiling point and melting point have
A semiempirical model has been developed for the estimation of the normal boiling points of organic compounds. The normal boiling point is calculated as the ratio of the enthalpy of boiling to the entropy of boiling. Both these values are estimated independently using a combination of additive group contribution and nonadditive molecular
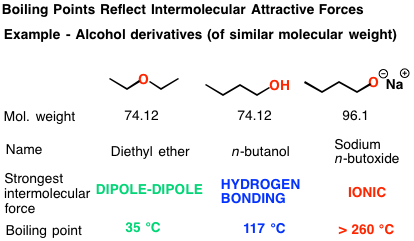
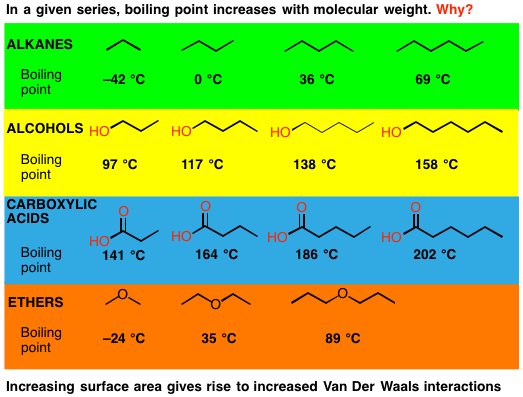
Boiling points of organic compounds depend on individual atoms or groups, polarizability of the molecule, length of carbon backbone, shape and branching of the molecule and their contribution to the structural environment in the molecule.
can be used for the determination of boiling point and for simple distillation of small volumes of liquid organic compounds. The apparatus also provides a safe method for determination of melting points. Keywords . Apparatus, Boiling Point, Melting Point . 1. Introduction . The determination of physical constants such as boiling point and melting point is among the key basic organic chemistry
MELTING POINT AND BOILING POINT OF ORGANIC COMPOUNDS—– ABSTRACT The melting point (MP) and the boiling point (BP) are probably the most widely used physical constant in the field of science. Determining the boiling point and the melting point of a compound helps you to characterize an unknown solid in a quick, easy and cheap way.
Just like with boiling points, the presence of polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points. The size of a molecule influences its melting point as well as its boiling point, again due to increased van …
The melting point helps to characterize new compounds. In this lab, the identity of an unknown organic compound will be determined by comparing its experimental melting point to those of a variety of known compounds.
Melting Point and Boiling Point of Organic Compounds Essay Sample Lab Report for CHM 152 Name Lab Partner(s) Abstract: The physical properties, including the boiling point, density, and refractive index were measured for unknown liquid #16 – Melting Point and Boiling Point of Organic Compounds Essay introduction.
Determination of boiling point of organic compounds – Free download as PDF File (.pdf), Text File (.txt) or read online for free. Scribd is the world’s largest …
Boiling Point: It identifies the physical properties of an organic compounds. It varies depending upon the surrounding environment. A boiling point of a liquid is high at high pressure and have lower boiling point when atmospheric pressure is low. There are several factors that would affect boiling point and they are stated below.
CHEM 333L Organic Chemistry Laboratory Revision 2.1 Steam Distillation of an Essential Oil In this laboratory exercise we will employ Steam Distillation to isolate an Essential Oil from
A method for estimating the boiling points of organic

Carbonyl Chemistry – Fundamentals
The boiling point of the solvent should be below the melting point of the solid to prevent the solid from coming out of solution as a liquid, typically called “oiling out”. The liquid -liquid equilibrium that occurs during oiling out can cause substantial impurities to dissolve in the desired compound • The solvent must be un- reactive with the solid solute • Co-solvent mixtures can be
The boiling point of a molecule is determined by its formula weight and the types of functional groups it contains. Arrange the following compounds in order of decreasing boiling points. Explain your reasoning. a) CH 3 CH 2 CH 3, CH 3 CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 CH 3, CH 3 CH 2 CH 2 CH 2 CH 3 b) CH 3 CH 2 CH 2 CH 3 or OH. Chemistry of Natural Substances – Organic Chemistry
Just like with boiling points, the presence of polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points. The size of a molecule influences its melting point as well as its boiling point, again due to increased van der Waals interactions between molecules.
The exact boiling point of the mixture depends upon the relative amounts of the compounds present. Figure 1 shows the relationship between boiling point and composition for a two-compound mixture of cyclohexane and toluene.
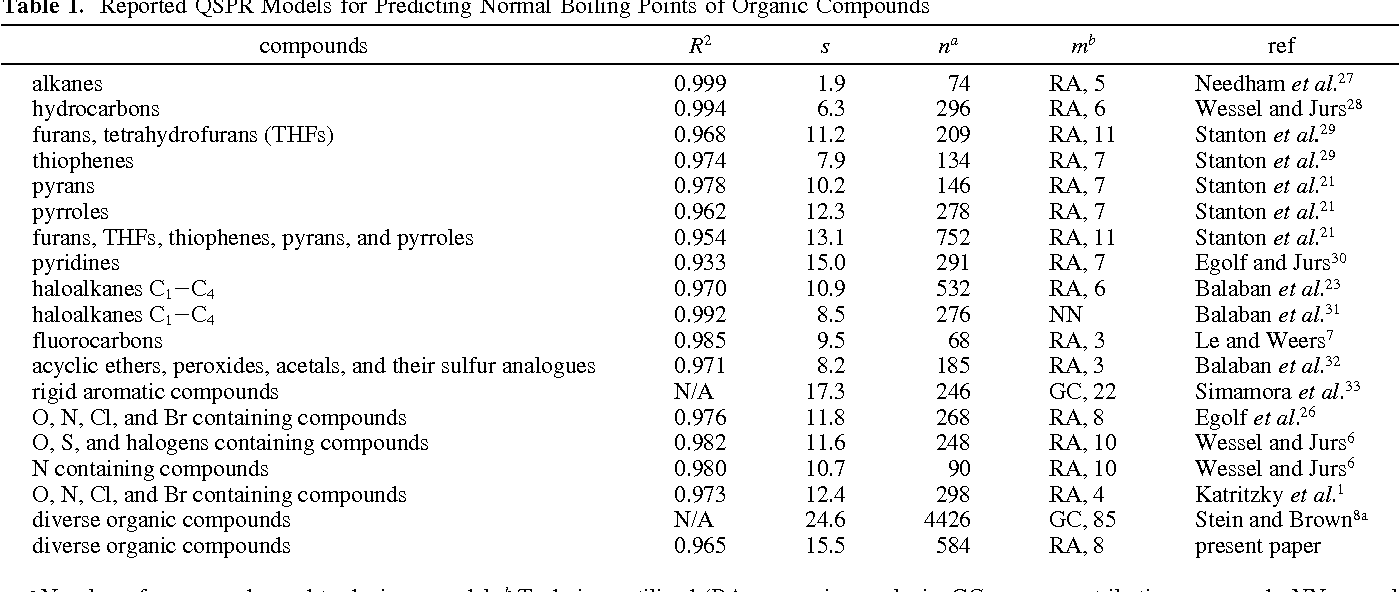
two descriptors for the prediction of boiling points for various other classes of organic compounds was investigated further by employing a diverse data set of 612 organic compounds …
The last compound, an isomer of octane, is nearly spherical and has an exceptionally high melting point (only 6º below the boiling point). Contributors William Reusch, Professor Emeritus ( Michigan State U. ), Virtual Textbook of Organic Chemistry
organic, organo-metallic, and a few inorganic compounds. This compendium is the third This compendium is the third in a series focusing on phase change enthalpies.

ORGANIC LABORATORY TECHNIQUES 5 5.1 BOILING POINT DETERMINATION The physical properties of a compound, such as melting point and boiling point can provide useful
Identification of Unknown Organic Compounds Introduction The identification and characterization of the structures of unknown substances are an important
The boiling point is used to characterize a new organic liquid, and knowledge of the boiling point helps to compare one organic liquid with another, as in the process of identifying an unknown organic …
Carbonyl Chemistry (12 Lectures) proton); therefore, they have lower boiling points than alcohols of similar molecular weight. ¥Aldehydes and ketones are hydrogen bond acceptors; this makes them have considerable solubilities in water. R C R ‘ O O H H H O H Ketones such as acetone are good solvents because they dissolve both aqueous and organic compounds Recall that acetone is a polar
estimation of boiling points of organic compounds russian journal of general chemistry vol. 79 no. 4 2009 779 commonly indicated.
parameters such as boiling point, vapor pressure, molecular weight, and size. EU EU directive 2004/42/EC defines VOCs as any organic compound having an initial boil-
The polarity of the carbonyl group and its higher basicity than alkenes lower the transition state energy of activation and therefore result in a faster rate. Fig.1
The trends in physical properties of organic compounds, such as melting point, boiling point, viscosity, flashpoint and solubility, can be deduced from their structure, including size, shape and any functional groups. These factors depend on the strength of the intermolecular forces present. In molecules (where atoms are connected by intramolecular covalent bonds), intermolec-ular forces may
23/04/2018 · This organic chemistry video tutorial provides a basic introduction into boiling point of organic compounds such as straight chain alkanes, branched alkanes, aldehydes, amines, alcohols, and …
Keywords Boiling point Melting point Molecular weight Polymeric compounds Molecular compounds Structure Comparison of organic and inorganic compounds—general features The standard boiling and melting points have been defined by IUPAC (Cox et al. 1982)as the temperature at which boiling/melting occurs under a pressure of 1 bar. When dealing with the points it is tacitly supposed …
VOC – volatile organic compounds present in paint formulations (either as a ingredient uniqueor as a a vapour pressure >0.01mm Hg at 21ºC or , b) an initial boiling point <250ºC measured at a standard pressure of 101.3 kPa. Note 1: Ammonia will be classified as a VOC . Note 2:
1 database found for boiling point. The NIST Chemistry WebBook contains: Thermochemical data for over 7000 organic and small inorganic compounds; thermochemistry data for over 8000 reactions; IR spectra for over 16,000 compounds; mass spectra for over 33,000 compounds; UV/Vis spectra for over 1600 compounds; electronic and vibrational spectra for over 5000 compounds; constants of …
Organic chemistry is the study of carbon compounds. Because this branch of chemistry covers Because this branch of chemistry covers such a large number of compounds, organic compounds are it is broken down into many
Boiling Point an overview ScienceDirect Topics
The boiling point of a molecule is determined by its formula weight and the types of functional groups it contains. Arrange the following compounds in order of decreasing boiling points. Explain your reasoning. a) CH
What are the trends in boiling point for three families of carbon compounds? Design The boiling points of twelve organic compounds (from three chemical families)
A semiempirical model has been developed for the estimation of the normal boiling points of organic compounds. The normal boiling point is calculated as the ratio of the enthalpy of boiling to the
Boiling Point of a liquid can be defined as the temperature at which the vapor presence of its become atmospheric pressure since the Boiling Point of a liquid depend on atmospheric pressure which varies from place to place hence boiling point of a substance also varies for expl. The Boiling Point of water at sea level is 100° C but it is lower on the heats where atmospheric pressure is less
EXPERIMENT 1 – Determination of the purity and identity of organic compounds by melting point and/or analytical thin layer chromatography PART A Melting points and mixed melting points.
boiling point of ethanol (78.5″) or of water ( 100″). In contrast, a 1.35: 1 mole mixture In contrast, a 1.35: 1 mole mixture of methanoic (formic) acid and water boils at 107.l0, which is higher than the boiling
1# # Lab:QualitativeOrganicAnalysis$ Written’by’ Danielle’M.’Solano’ Department’of’Chemistry’&Biochemistry’ California’State’University
D.M. Collard 2007 TOPIC 2. ORGANIC COMPOUNDS (Chapter 2) L OBJECTIVES 1. Describe the structure of N, O and halogen containing functional groups: formal charges, hybridization, VSEPR
ORGANIC LABORATORY TECHNIQUES 5 5.1 BOILING POINT DETERMINATION The physical properties of a compound, such as melting point and boiling point can provide useful information which can help in the identification of a sample or to establish its purity. Since the boiling point of an unknown sample under the same conditions (e.g. same pressure) is a constant, a measured boiling point …
Melting Point and Boiling Point of Organic Compounds Bongo, Sayre, J1 1Student, Organic Chemistry 1 Laboratory / B11, School of Chemical Engineering, Chemistry and Biotechnology, Mapúa Institute of Technology ABSTRACT The melting point of a substance is the temperature at which the material changes from a solid to a liquid state while the boiling point is the temperature at which it … – image of cleaver boiler model cfce500 manual compounds with these atoms, as we will show later in this chapter. Bond Polarity (3.1C) With the exception of the protonated amines and alcohols just mentioned, all of the organic
ie00031a030_si_001.pdf (2.93 MB) A semiempirical model has been developed for the estimation of the normal boiling points of organic compounds. The normal boiling point is calculated as the ratio of the enthalpy of boiling to the entropy of boiling. Both these values are estimated
Organic Chemistry: Techniques and Transformations 4 Summary 1. The melting point of a compound is the temperature range at which a solid transforms into a liquid (the temperature at which the first drop of liquid appears until
E24 PURIFICATION OF ORGANIC COMPOUNDS Distillation, recrystallisation, melting and boiling point determination THE TASK To learn the main techniques of purifying organic compounds.
In this experiment, melting point and boiling point of organic compounds is to be determined. The main objective of the experiment is to determine the effects of various factors like intermolecular forces of attraction, purity, and branching to the melting and boiling point range of organic compounds. Thus, the structural theory was given an emphasis in the experiment as well as in this paper
The boiling point of organic compounds can give important information about their physical properties and structural characteristics. Boiling point helps identify and characterise a compound. A liquid boils when its vapour pressure is equal to the atmospheric pressure. Vapour pressure is determined by the kinetic energy of a molecule.
Some examples of Low Boiling point organic compounds are below: Group 1 Low boiling point organic vapours with an Exposure Standard (ES) of less …
EXPERIMENT 1 (Organic Chemistry I) Melting Point Determination . Purpose. a) Determine the purity of a substance using melting point as physical property . b) Identify an unknown compound using its melting point . c) Identify an unknown compound using mixture melting point . d) Learn how to obtain an accurate melting point using a Mel-Temp apparatus . Discussion Identifying an unknown compound
A method for estimating the boiling points of organic compounds from their melting points
Relationships between Melting Point and Boiling Point of
Boiling Point of an Organic Compound (Procedure) Class
Prediction of boiling points of organic compounds by QSPR
LIBRARY OF PETROLEUM PRODUCTS ALS Global

DETERMINATION OF MELTING POINTS Westminster College
Boiling points of organic compounds (video) Khan Academy

EXPERIMENT 1 Determination of the purity and identity of
https://simple.wikipedia.org/wiki/Chemical_nomenclature
1 database found for boiling point. SRDATA at NIST
– Analysis of Volatile Organic Compounds in Waterborne
Dictionary of Organic Compounds 26.2

Organic Lab Manuals for Ventura College – Home
Normal Boiling Points for Organic Compounds Correlation
10 replies on “Organic compounds boiling point pdf”
Leave a CommentThe trends in physical properties of organic compounds, such as melting point, boiling point, viscosity, flashpoint and solubility, can be deduced from their structure, including size, shape and any functional groups. These factors depend on the strength of the intermolecular forces present. In molecules (where atoms are connected by intramolecular covalent bonds), intermolec-ular forces may
Analysis of Volatile Organic Compounds in Waterborne
Apparatus Boiling Point Melting Point sapub
organic, organo-metallic, and a few inorganic compounds. This compendium is the third This compendium is the third in a series focusing on phase change enthalpies.
2.5 Physical properties of organic compounds Chemistry
23/04/2018 · This organic chemistry video tutorial provides a basic introduction into boiling point of organic compounds such as straight chain alkanes, branched alkanes, aldehydes, amines, alcohols, and …
Apparatus Boiling Point Melting Point sapub
boiling point of ethanol (78.5″) or of water ( 100″). In contrast, a 1.35: 1 mole mixture In contrast, a 1.35: 1 mole mixture of methanoic (formic) acid and water boils at 107.l0, which is higher than the boiling
2.5 Physical properties of organic compounds Chemistry
1 database found for boiling point. SRDATA at NIST
EXPERIMENT 1 – Determination of the purity and identity of organic compounds by melting point and/or analytical thin layer chromatography PART A Melting points and mixed melting points.
Dictionary of Organic Compounds 26.2
Boiling Points of Organic Compounds SchoolWorkHelper
Organic Chemistry: Techniques and Transformations 4 Summary 1. The melting point of a compound is the temperature range at which a solid transforms into a liquid (the temperature at which the first drop of liquid appears until
Carbonyl Chemistry – Fundamentals
Estimation of the Normal Boiling Point of Organic Compounds
The polarity of the carbonyl group and its higher basicity than alkenes lower the transition state energy of activation and therefore result in a faster rate. Fig.1
TOPIC 2. ORGANIC COMPOUNDS (Chapter 2)
LIBRARY OF PETROLEUM PRODUCTS ALS Global
The boiling point of a molecule is determined by its formula weight and the types of functional groups it contains. Arrange the following compounds in order of decreasing boiling points. Explain your reasoning. a) CH
Boiling Points of Organic Compounds SchoolWorkHelper
Structure-related melting and boiling points of inorganic
ORGANIC LABORATORY TECHNIQUES 5 5.1 BOILING POINT DETERMINATION The physical properties of a compound, such as melting point and boiling point can provide useful
2.5 Physical properties of organic compounds Chemistry
Boiling Point an overview ScienceDirect Topics
DETERMINATION OF MELTING POINTS Westminster College
ie00031a030_si_001.pdf (2.93 MB) A semiempirical model has been developed for the estimation of the normal boiling points of organic compounds. The normal boiling point is calculated as the ratio of the enthalpy of boiling to the entropy of boiling. Both these values are estimated
Estimation of Boiling Points of Organic Compounds at
A method for estimating the boiling points of organic
Comments are closed.